What Is Immune System And Its Function – Antimicrobial resistance and plasmid profile analysis of Salmonella enteritidis in Siberia and the Far East of Russia between 1990 and 2017
Open Access Policy Institutional Open Access Program Special Issues Guidelines Editorial Process Research and Publication Ethics Article Processing Fees Awards Testimonials
What Is Immune System And Its Function

All articles published by are made immediately available worldwide under an open access license. No special permission is required to reuse the published article in whole or in part, including figures and tables. For articles published under the Open Access Creative Commons CC By License, any part of the article may be reused without permission, provided the original article is clearly cited. For more information, please refer to https:///openaccess.
The Immune System Poster
Feature papers represent the most advanced research with significant potential for high impact in the field. A feature paper should be a truly original article that covers multiple techniques or approaches, provides perspectives for future research directions, and describes potential research applications.
Feature papers are submitted at the personal invitation or recommendation of scientific editors and must receive positive feedback from reviewers.
Editor’s Choice articles are based on recommendations from scientific editors of journals around the world. The editors select a few articles recently published in the journal that they believe will be of particular interest to readers or in relevant research areas. It aims to provide a snapshot of some of the most exciting work published in the journal’s various research areas.
By Catherine Elizabeth Bline by Catherine Elizabeth Bline Skillitt Preprints.org Google Scholar * and Mark W. Hall Mark W. Hall Skillitt Preprints.org Google Scholar
The Central Role Of The Gut
Received: 29 August 2021 / Revised: 13 September 2021 / Accepted: 22 September 2021 / Published: 25 September 2021
The inflammatory response in pediatric sepsis is highly dynamic and includes both inflammatory and inflammatory factors including innate and adaptive immunity. While a pro-inflammatory response is responsible for the early clinical signs and symptoms of sepsis, a concurrent compensatory anti-inflammatory response is often the result of a latent, but highly clinically relevant, form of acquired immunodeficiency. When severe, this is called “immunoparalysis” and is associated with an increased risk of nosocomial infection, prolonged organ dysfunction, and death. This review focuses on the pathophysiology and clinical implications of over- and under-active immune function in septic children. Host-, disease-, and treatment-specific risk factors for immunoparalysis are reviewed, along with immune phenotype-specific approaches to immunomodulation in pediatric sepsis that are currently the subject of clinical trials.
Sepsis represents an uncontrolled host response to infection that results in organ failure when severe. Sepsis is an important public health problem and a significant cause of morbidity and mortality in children worldwide [ 1 , 2 ]. Timely recognition of sepsis and provision of fluid resuscitation and antibiotics have improved sepsis outcomes over the past two decades [3, 4], with more than 7000 children dying of sepsis annually in the United States alone [5]. The pathophysiology of the immune response in sepsis is an attractive target for the development of new therapies to improve outcomes in critically ill children with sepsis.

Historically, most studies of sepsis interventions have focused on the use of supportive care (eg, antibiotics, intravenous fluids, and other bundle components) or anti-inflammatory therapies. Indeed, although sepsis is triggered by the initial infectious insult, much of the damage to the host is not caused directly by the pathogen but by the host’s dysregulated inflammatory response that may persist even after the initial infection has been controlled. We now know that the immune response in sepsis is highly dynamic, with many children displaying a compensatory anti-inflammatory response that represents a clinically significant, latent form of acquired immunodeficiency. Understanding the variability of the immune response in children with sepsis will allow the development of new, personalized approaches to sepsis treatment.
The Immune System: Cells, Tissues, Function, And Disease
The immune system is made up of two arms: the innate and the adaptive immune system (Figure 1). These two systems include immune cells and mediators that function to detect and eliminate harmful pathogens and promote remodeling of injured tissue. The innate immune system recognizes a broad class of pathogen-associated molecular patterns (PAMPs) through constitutively expressed PAMP receptors, such as Toll-like receptors (TLRs) including the lipopolysaccharide (LPS) receptor TLR4 (6).
It is usually the first cellular arm of the immune system to respond to infection. Activated innate immune cells, including neutrophils, monocytes, macrophages, and dendritic cells, initially produce proinflammatory chemokines and cytokines that favor the local environment to fight infection and recruit other immune cells to the affected area. When this response spreads to the systemic circulation, however, the clinical findings of sepsis become apparent. Examples of proinflammatory cytokines commonly produced by innate immune cells include interleukin (IL)-1β and tumor necrosis factor (TNF)α. In addition to cytokine production, most innate immune monocytes are capable of phagocytosis and intracellular killing of pathogens. Antibiotic peptides from the digested pathogen are then loaded onto class II major histocompatibility complex (MHC) molecules such as human leukocyte antigen (HLA)-DR for presentation to the adaptive arm of the immune system.
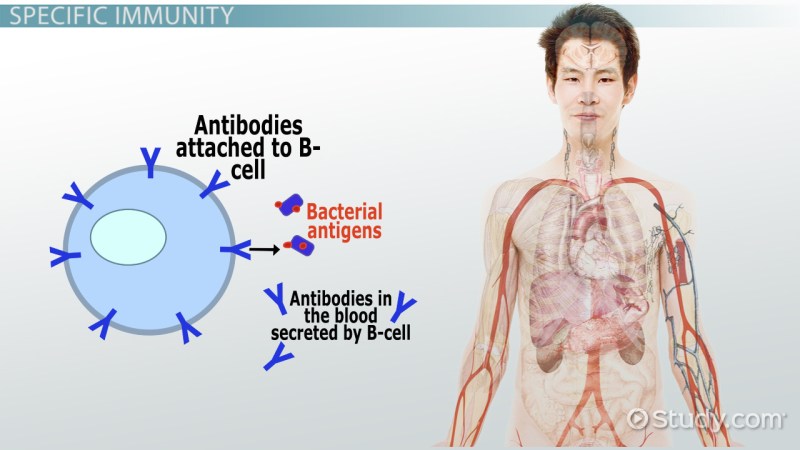
The adaptive immune system consists of lymphocytes that, while slower to respond to infection, are more specific for a given pathogen. The adaptive immune system is also capable of generating long-lived memory cells, allowing for a more rapid and efficient response to repeated infections. Adaptive immune cells include T lymphocytes and B lymphocytes. T cells include CD8+ cytotoxic T cells that effect cellular killing through the release of lytic enzymes [6] and CD4+ helper T cells that produce cytokines that modulate the activity of other immune cells and parenchymal cells. Helper T cells can differentiate from naïve CD4+ cells into one of several subsets of helper T cells depending on the cytokine environment in which they are activated. While the many subtypes of helper T cells are beyond the scope of this review, the following subtypes are worth discussing: Th1, Th2, Th17, and regulatory T cells (Tregs) [7]. Th1 cells produce proinflammatory mediators such as IL-2 and interferon (IFN)γ, while Th17 cells produce the even more potent proinflammatory cytokine IL-17. Th2 cells produce characteristic mediators of anti-inflammatory and allergic responses, including IL-4, IL-5, and IL-10, and are important in promoting B cell maturation and subsequent antibody production. Tregs are more effectively anti-inflammatory, producing IL-10 and transforming growth factor (TGF)-β.
Both arms of the immune system can produce mediators that are part of the systemic inflammatory response syndrome (SIRS). It is characterized by the production of proinflammatory cytokines such as TNFα, IL-1β, and IFNγ [8]. An uncontrolled, severe SIRS response can result in fever, capillary leakage, organ dysfunction, and death [9] (Figure 2). Almost concurrently with the initial SIRS response, the host also experiences a compensatory anti-inflammatory response syndrome (CARS) that acts as a negative feedback mechanism to reduce inflammation-related damage. A CARS response is clinically latent but can be detected in the laboratory with specific testing. Findings consistent with a CARS response include decreased leukocyte cytokine production capacity; decreased expression of HLA-DR on the cell surface of circulating monocytes; systemic lymphopenia with lymphocyte apoptosis in the spleen; and an increase in systemic levels of anti-inflammatory cytokines [10, 11]. Interestingly, this increase in serum cytokines such as IL-10 is often accompanied by increased levels of pro-inflammatory biomarkers including IL-6 and IL-8. While the concomitant increase in systemic proinflammatory cytokines and decreased leukocyte cytokine production capacity may seem paradoxical, it is important to understand that proinflammatory mediators are abundantly produced by injured parenchymal cells and stressed vascular endothelium. In some cases these may be the source of systemic inflammation rather than circulating leukocytes.
Cells Of The Immune System • Ibiology
When severe, the CARS response is termed “immunoparalysis” and may be considered a clinically relevant form of immunodeficiency. Several diagnostic methods have been used to define immunoparalysis. To date, the largest body of data on immunoparalysis in critically ill children with infection has measured the ability of subjects’ whole blood to produce TNFα upon ex vivo stimulation with LPS. Blood samples from healthy infants should strongly produce TNFα after exposure to LPS. Children with immunoparalysis show a significant reduction in this TNFα production capacity or “TNFα response”. In the most commonly used assay, a response < 200 pg/mL after stimulation of whole blood with 500 pg/mL LPS for four hours is associated with frequent mortality, increased nosocomial infection risk, and prolonged organ dysfunction in septic children. 10, 12, 13, 14, 15]. In children with severe influenza infection, the inclusion of antiviral retinoic acid-inducible gene-I (RIG-I) pathway stimulation testing may add prognostic value compared to TLR4 pathway stimulation alone [15].
Flow cytometry has been associated with adverse outcomes in septic adults [16, 17, 18]. This has also been observed in septic children [10], although even mild suppression is associated with adverse outcomes [19]. This method of quantification of HLA-DR expression is at risk of bias due to lot-to-lot variability in fluorochromes and differences in cytometer settings. An alternative flow cytometric method involves using standard beads
What is endocrine system and its function, what is an enzyme and its function, what is a nucleus and its function, what is skeletal system and its function, what is epiglottis and its function, what is lymphatic system and its function, what is a transformer and its function, what is nervous system and its function, what is immune system function, immune system structure and function, what is platelets and its function, immune system function and organs