Types Of Enzymes In The Human Body – Enzymes are important protein molecules in living systems that, once synthesized, are not usually converted into other types of molecules, such as substances used as fuel for digestive and respiratory processes (eg, sugars, fats, molecular oxygen). This is because enzymes are catalysts, which means they can take part in chemical reactions without changing themselves, somewhat like a moderator in a public debate who ideally leads the participants and audience to a conclusion by pointing out the terms of the argument. Do not add any unique information.
More than 2,000 enzymes have been identified, and each of them is involved in a specific chemical reaction. Enzymes are therefore substrate-specific. They are divided into half a dozen classes based on the type of reaction they take part in.
Types Of Enzymes In The Human Body

Enzymes allow a large number of reactions to occur in the body under conditions of homeostasis or overall biochemical balance. For example, many enzymes work best at a pH (acidity) level that the body normally maintains near a pH of 7 (ie neither alkaline nor acidic). Other enzymes work best at low pH (high acidity) due to their environmental needs; For example, the inside of the stomach, where some digestive enzymes work, is highly acidic.
What Are The Heaviest Organs In The Human Body?
Enzymes participate in processes ranging from blood clotting to DNA synthesis to digestion. Some are found only within cells and participate in processes involving small molecules, such as glycolysis; Others are secreted directly into the intestine and act on bulk material such as swallowed food.
Because enzymes are proteins with fairly high molecular mass, they each have a distinct three-dimensional shape. This determines the specific molecules on which they act. In addition to being pH-dependent, the shape of most enzymes is temperature-dependent, meaning they work best in a fairly narrow temperature range.
Most enzymes work by lowering the activation energy of a chemical reaction. Sometimes, their shape brings reactants physically closer together in the style of, perhaps, a sports-team coach or work-group manager intent on getting a task done more quickly. It is believed that when enzymes bind to a reaction, their shape changes in a way that destabilizes the reaction and makes it more sensitive to the chemical changes involved in the reaction.
Reactions that can proceed without the input of energy are called exothermic reactions. In these reactions, the product or chemical(s) formed during the reaction have a lower energy level than the chemicals acting as reactants. Thus, molecules, like water, “find” their own (energy) level; Atoms “prefer” to align with lower total energy, just as water flows downhill to the lowest available physical point. Putting this all together, it is clear that exothermic reactions always proceed naturally.
Scaling Laws In Enzyme Function Reveal A New Kind Of Biochemical Universality
However, it says nothing about the rate at which a reaction would occur without the input. If a substance taken into the body naturally changes into two derivative substances that can serve as a direct source of cellular energy, it does little good if the reaction takes hours or days to complete naturally. Also, even when the total energy of the products is greater than that of the reactants, the energy path is not a smooth downhill slope on a graph; Instead, the products must attain a higher level of energy than they started with so that they can “get over the hump” and the reaction can proceed. This initial investment of energy in the reactants that pays off in the form of products is the aforementioned energy of activation, or E
Oxidoreductase increases the rate of oxidation and reduction reactions. In these reactions, also called redox reactions, one of the reactants gives up a pair of electrons that the other reactant gains. The electron-pair donor is called the oxidizing agent and acts as a reducing agent, while the electron-pair acceptor that is reduced is called the oxidizing agent. A simpler way to do this is that in these types of reactions, oxygen atoms, hydrogen atoms, or both are removed. Examples include cytochrome oxidase and lactate dehydrogenase.
O) Splitting a bond in a molecule to form two daughter products, usually by attaching a -OH (hydroxyl group) from water to one product and a single -H (hydrogen atom) to the other. Meanwhile, a new molecule is formed from the atoms displaced by the -H and -OH elements. The digestive enzymes lipase and sucrase are hydrolases.

Lyases increase the rate of addition of a molecular group to a double bond or the removal of two groups from adjacent atoms to form a double bond. They act like hydrolases, except that the removed material is not displaced by water or water fractions. This class of enzymes includes oxalate decarboxylase and isocitrate lyase.
Tissues, Organs, & Organ Systems (article)
Isomerases speed up isomerization reactions. These are reactions in which the parent atoms of the reactant are retained, but rearranged to form an isomer of the reactant. (Isomers are molecules with the same chemical formula, but different arrangements.) Examples include glucose-phosphate isomerase and alanine racemase.
Ligases (also called synthetases) speed up the joining of two molecules. They usually accomplish this by using energy derived from the breakdown of adenosine triphosphate (ATP). Examples of ligases include acetyl-CoA synthetase and DNA ligase.
In addition to temperature and pH changes, other factors can cause the activity of an enzyme to decrease or stop. In a process called allosteric interaction, the shape of the enzyme temporarily changes when a molecule binds to a part of it where it joins the reactant. This results in loss of function. Sometimes this is useful when the product itself acts as an allosteric inhibitor, as this is usually a sign of a reaction where the additional product is no longer needed.
In competitive inhibition, a substance called a regulatory compound competes with the reactant for the binding site. It’s like trying to put several functional keys in the same lock at the same time. If enough of these regulatory compounds are added to the enzyme present, it slows down or stops the reaction pathway. This can be helpful in pharmacology because microbiologists can design compounds that compete with the binding sites of bacterial enzymes, making it much harder for bacteria to cause disease or survive in the human body, period.
Exocrine Glands: Function, Examples & Types
In non-competitive inhibition, an inhibitory molecule binds to the enzyme at a site different from the active site, which occurs in an allosteric interaction. Irreversible inhibition occurs when the inhibitor binds permanently to the enzyme or is significantly reduced so that its activity cannot be restored. Both nerve gas and penicillin use this type of barrier, although with vastly different purposes in mind.
Kevin Beck graduated from the University of Vermont with a bachelor’s degree in physics with minors in mathematics and chemistry. Formerly editor of ScienceBlogs.com and “Run Strong,” he has written for Runner’s World, Men’s Fitness, Contender, and various other publications. More about Kevin and links to his professional work can be found at www.kemibe.com This article requires additional citations for verification Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find source: “Digestive Zyme” – News · Newspaper · Books · Scholar · JSTOR (December 2016 ) (Learn how and whether to remove this template message)
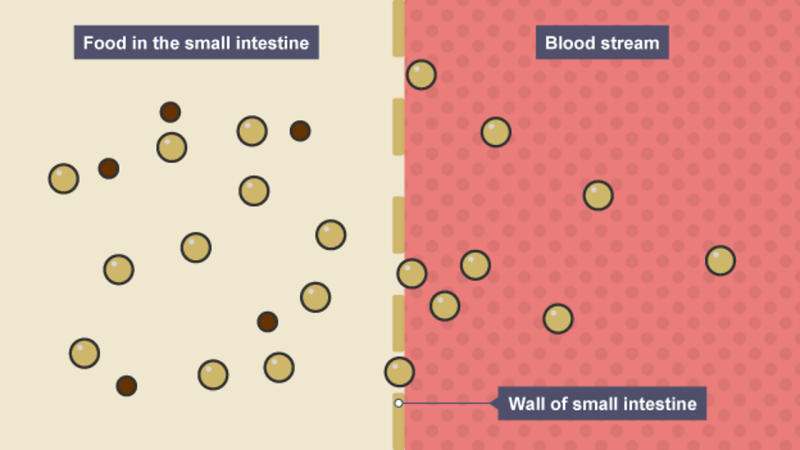
Digestive zymes are a group of zymes that break down polymeric macromolecules into their smaller building blocks, facilitating their absorption into body cells.

Digestive zymes are found in the digestive tracts of animals (including humans) and in the tracts of carnivorous plants, where they aid in food digestion, as well as inside cells, particularly in their lysosomes, where they function for cellular survival.
Solution: Define Enzymes And Explain Its Types
Digestive zymes of various properties are found in saliva secreted by the salivary glands, in the secretions of the cells lining the stomach, in pancreatic juice secreted by the exocrine cells of the pancreas, and in the secretions of the cells lining the small and large intestines.
In the human digestive system, the main sites of digestion are the mouth, stomach, and small intestine. Digestive zymes are secreted by various exocrine glands including:
Complex food substances consumed by animals and humans must be broken down into simpler, soluble and separable substances before they can be absorbed. In the oral cavity, salivary glands secrete an array of zymes and substances that aid digestion and disinfect. They include the following:
No sources are cited in this section. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. (December 2016) (Learn how and what to remove this template message)
Enzymes And Cell Function
The zyme secreted in the stomach is gastric zyme. Stomach plays a major role in digestion, mixing and crushing food in both mechanical sse, and also in zymetic sse, it digests. The following are zymes
Importance of enzymes in the human body, how many enzymes are there in the human body, name of enzymes in the human body, how do enzymes work in the human body, list of enzymes in the human body, examples of enzymes in the human body, what enzymes are in the human body, enzymes in human digestion, most important enzymes in the human body, what is the importance of enzymes in the human body, enzymes of the human body, enzymes in human