What Acid Rain Does To The Environment – The cycle of processes by which water circulates between Earth’s oceans, atmosphere, and land, including precipitation as rain and snow, runoff in streams and rivers, and return to the atmosphere by evaporation and evaporation.
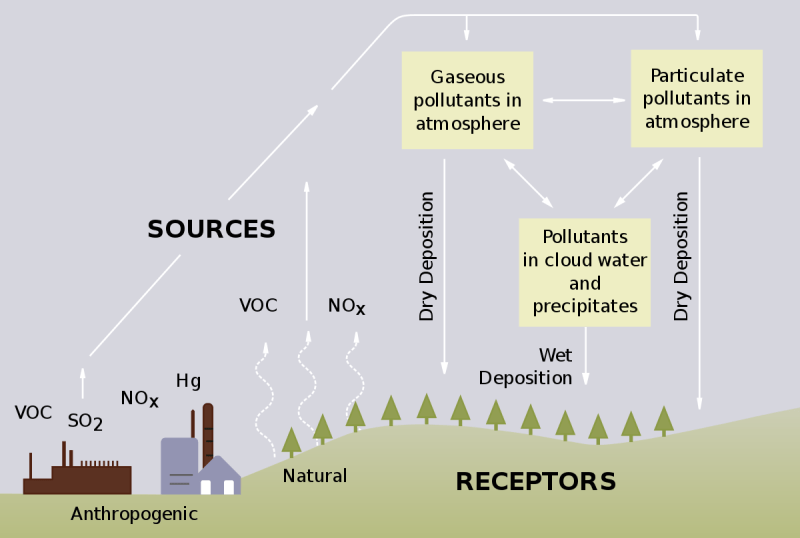
Acid rain is the result of sulfur dioxide and nitrogen oxides emitted by various industries mixing with the air, then reacting with water, oxygen and other chemicals in the atmosphere.
What Acid Rain Does To The Environment
Without the addition of these pollutants, the acid level in rain is usually between 5.0 and 5.5 on the pH scale, which is only slightly acidic. When combined with SO₂ and NOx, acidity can increase up to tenfold, dropping to around 4.0 on the pH scale. Acids trapped in rain can only be neutralized by precipitation on alkaline surfaces such as limestone (calcium carbonate). In some lucky areas, limestone is also part of the soil, so those areas are part of the earth’s ability to reduce acid in rain and snow. Unfortunately, in areas not so fortunate to have limestone, acid rain can damage the environment.
Acid Rain Virtual Lab Worksheet
Plants, fish, and aquatic life are negatively affected by acid rain. It has also been linked to declining wildlife populations, dangerous lakes and streams, and human health threats. This can damage plant leaves and stunt plant growth. It has been shown to affect the plant’s ability to fight cold and disease. It also makes the soil more acidic for plants to dissolve essential minerals.
For fish, acid rain disrupts the levels of naturally occurring minerals in their bodies, which affects their reproductive systems, causing females to be unable to produce eggs. In acidic water, their gills can become sticky, and eventually become unable to take in oxygen from the water. Acid rain also leaches aluminum from soil. When it is introduced into water systems with fish, it makes the environment less hospitable to aquatic life. Different forms of water-based species can withstand different levels of acid. For example, frogs can survive at a pH level of 4.0, while clams and mussels can only handle a level of 6.0.
Since the 1970s and 1980s, there has been a decrease in acid rain in some areas, mainly Europe and North America. This is largely due to regulations to minimize its occurrence, such as the US Clean Air Act of 1970 and the Canada-United States Air Quality Agreement in 1991. This is not true even in areas where many pollutants are released, such as in China. and India. In those areas, acid rain is still affecting people, animals and water resources. Acidic deposition refers to acids and acidifying substances, including sulfuric and nitric acid and ammonium, derived from atmospheric emissions of sulfur dioxide, nitrogen oxides, and ammonia, respectively. Sulfur dioxide and nitrogen oxides are mostly released by burning fossil fuels, while ammonia emissions are mainly related to agricultural activities. Atmospheric deposition delivers acids and acidifying compounds to the Earth’s surface, with adverse ecological effects occurring largely in areas sensitive to high emissions in eastern North America, northern and central Europe, and southern China. Acidic storage in leaves, and forest soils, is affected by reducing available calcium and magnesium and increasing soil water-soluble inorganic aluminum concentrations, resulting in health effects on trees. Acidic deposition has degraded water quality in eastern North America and Europe by lowering pH levels (ie increasing acidity) and acid-neutralizing capacity, and increasing dissolved inorganic aluminum concentrations. Many surface waters affected by acidic deposition in acid-sensitive areas exhibit chronic and/or episodic (i.e., short-term) acidification that has reduced species diversity and abundance of aquatic life. Most attention has been focused on fish, but entire food webs have been negatively affected. Recent reductions in sulfur dioxide and nitrogen oxide emissions in North America and Europe have reduced acid deposition with some improvements in surface water quality. However, recovery of forest soils and biota has generally been slow. This chapter will illustrate the effects of acidic deposition using examples from research conducted in the Hubbard Brook Ecosystem Study.
For more than five decades, research at HBEF has provided insight into the effects of air pollution on the structure and function of ecosystems. Note that acidic deposits were first identified in North America at Hubbard Brook, NH (Likens et al., 1972). When first recognized in the 1960s and 1970s, acidic deposits were viewed as a simple problem that was limited in scope. It is now clear that acids and acidifying compounds enter remote unmanaged ecosystems by long-range atmospheric transport and deposition. These air pollutants are transported through soil to surface water, resulting in a range of complex and adverse ecosystem effects. In this chapter, information is synthesized on the sources and patterns of acid deposition, the effects of atmospheric sulfur and nitrogen deposition on sensitive forests and freshwater resources, and ecosystem recovery from emission control programs and mitigation strategies. These effects are largely illustrated using longitudinal research conducted at HBEF.
Acid Rain In The Adirondacks: An Environmental History
Acidic deposits include sulfuric and nitric acids derived from sulfur dioxide and nitrogen oxides, respectively, and ammonium resulting from the emission of ammonia. A detailed description of atmospheric deposition at Hubbard Brook and the interaction of atmospheric deposition with the forest canopy is provided in another chapter of this volume (see Atmospheric Inputs chapter). Sulfur dioxide and nitrogen oxides produced by human activities are emitted into the atmosphere by burning fossil fuels, while ammonia is mostly a result of agricultural activities. Once these compounds enter ecosystems, they can acidify soils and surface waters, bringing about a series of biochemical and ecological changes, discussed below. The term acidic deposition covers all types of acids and acidifying compounds that are transported from the atmosphere to the Earth’s surface, including gases, particles, rain, snow, cloud water, and fog. Acidic deposits occur as wet deposits that include rain, snow, hail, or hail; As a dry deposit, which consists of atmospheric particles or gases; or as cloud or fog deposits, which are more common at higher elevations and in coastal areas. Wet deposition is well characterized by monitoring at more than 270 National Atmospheric Deposition Program (NADP; https://nadp.slh.wisc.edu/ ) sites in the United States. In contrast, dry deposition is highly dependent on topography, climatic conditions, and vegetation. . characteristics, which can vary markedly over short distances in complex terrains. As a result, dry deposits are poorly characterized and highly uncertain. In the US, dry deposition is estimated through the Clean Air Status and Trends Network (CASTNet; http://www.epa.gov/castnet/), which includes about 90 sites.
Sulfuric and nitric acids lower the pH of rain, snow, soil, lakes and streams. In 1964–65 when the first measurements of pH of bulk deposition were made in Hubbard Brook, values ranged from 4.0 to 4.3, with an annual volume weighted value of 4.2. These values were 8 to 16 times more acidic than background conditions (pH ~ 5.2). In 2016–17, the annual average pH value of bulk deposition in Hubbard Brook was 5.1, which is only slightly more acidic than background conditions. These high pH values reflect a decline in emissions and acid deposition over the past five decades (see below).
Note that the acidity of rain is determined not only by acidic pollutants (sulfur dioxide, nitrogen oxides) but also reflects the balance between the concentration of acidic pollutants and acidic neutral bases, such as calcium and magnesium from airborne soil particles and acidic pollutants originating from ammonia. emissions. In many locations in Asia, high concentrations of sulfate and nitrate in precipitation are not accompanied by low pH due to pH buffering by high concentrations of calcium and ammonia.

Acidic deposition trends in eastern North America, Europe, and eastern Asia mirror emission trends in the atmospheric source region or airshed. An atmospheric source area or airshed is a spatial area that supplies air pollutants that contribute to atmospheric deposition for a given location. Atmospheric source areas can encompass emission sources several hundreds of kilometers from deposition sites. Long-term data from Hubbard Brook show decreasing concentrations of sulfate in bulk deposition since measurements began in the mid-1960s, consistent with decreases in sulfur dioxide emissions, and decreases in nitrates since the early 2000s due to decreases in nitrogen oxide emissions. input chapter). Based on these long-term data, a strong positive correlation exists between sulfur dioxide and nitrogen oxide emissions in the United States and sulfate concentrations and nitrate concentrations in wet or bulk deposition in Hubbard Brook (Figure 1). These observations suggest a cause and effect relationship between emissions of sulfur dioxide and nitrogen oxides and atmospheric deposition of sulfate and nitrate. Note that the correlation between sulfur dioxide emissions and sulfate concentrations in bulk and wet deposits suggests that current concentrations of sulfate are close to measurements made in remote areas that have been used as estimates of pre-industrial deposits (Figure 1). While nitrate concentrations have decreased significantly, they remain somewhat elevated. Furthermore, projections for future emissions control programs, such as the Affordable Clean Power Plan,
Acid Rain: Definition, Causes And Effects
How does acid rain damage the environment, what does acid rain do to the environment, why is acid rain a problem to the environment, how is acid rain harmful to the environment, how does acid rain affects the environment, how does acid rain affect our environment, what impact does acid rain have on the environment, acid rain and the environment, harmful effects of acid rain on the environment, why is acid rain harmful to the environment, effects of acid rain to the environment, what causes the acid rain