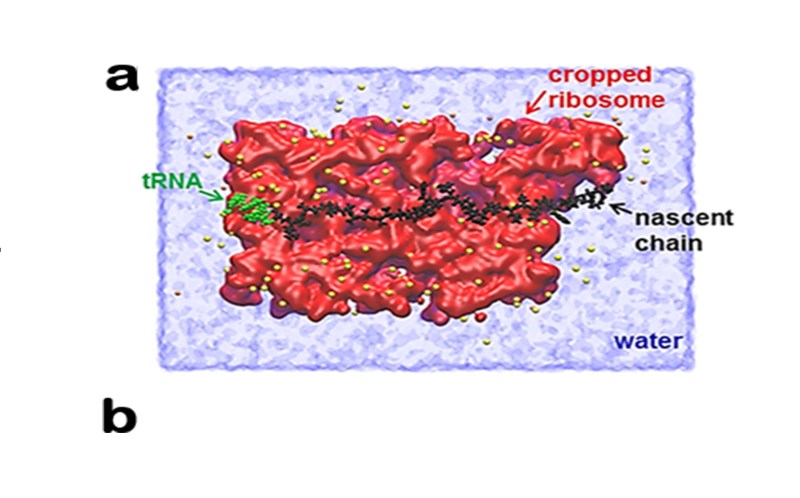
This Is The Site Of Protein Synthesis In The Cell – The process of protein synthesis translates the codons (nucleotide triplets) of messenger RNA (mRNA) into 20-character codes of amino acids that build the polypeptide chain of proteins. The process of mRNA translation proceeds from the 5′-end to the 3′-end as the polypeptide chain is formed from the amino-terminal (N-end) to the carboxyl-terminal (C -end). There is almost no significant difference between the process of protein synthesis in prokaryotes and eukaryotes, however, there is a significant difference in the structure of mRNA – prokaryotes usually have multiple regions (polycistronic mRNA), but eukaryotic mRNAs have only one region. monocistronic mRNA).
In most respects, the process in eukaryotes follows the same simple protein synthesis process as in prokaryotes. However, there are specific differences that can be highlighted. For example, one major difference is that in prokaryotic cells the transcription process begins before translation is complete. This relationship is determined because prokaryotes do not have a nuclear membrane and therefore there is no physical separation of the two phases.
This Is The Site Of Protein Synthesis In The Cell

The first step in protein synthesis is initiation, which involves the assembly of translational system components and precedes the formation of peptide bonds. The components involved in the first step of protein synthesis are:
Protein Synthesis Flowchart
Two mechanisms are involved in the recognition of the nucleotide sequence (AUG) by the ribosome, which actually initiates translation:
Translation elongation is secondary to the protein synthesis process. During the elongation process of the polypeptide chain, amino acids are added to the carboxyl end of the protein chain and the ribosome moves from the 5′-end to the 3′-end of the mRNA. In prokaryotes, delivery of aminoacyl-tRNA to the ribosomal A site facilitates the elongation of EF-Tu-GTP and EF-Ts, and requires GTP hydrolysis. In eukaryotes, similar elongation factors are EF-1α-GTP and EF-1βγ. Both EF-Ts (in prokaryotes) and EF-1βγ (in eukaryotes) function as nucleotide exchange factors.
Peptidyl-transferase is an important enzyme that catalyzes the formation of peptide bonds. Enzymatic activity is found to be within the 23S rRNA found in the large ribosomal subunit. Because this rRNA catalyzes the reaction of building a polypeptide, it is called a ribozyme.
The transfer RNA in the P site carries the currently synthesized polypeptide, while in the A site there is a tRNA, which binds to a single amino acid. After the formation of a peptide bond between the polypeptide and the amino acid, the newly formed polypeptide binds to the tRNA at the A site. After this step is completed, the ribosome transfers 3 nucleotides to the 3′ end of the mRNA. This process is known as translocation – in prokaryotes, it requires the participation of EF-G-GTP and GTP hydrolysis, while eukaryotic cells use EF-2-GTP and GTP hydrolysis again. During translocation, the uncharged tRNA moves from the P to the E site and the peptidyl-tRNA moves from the A site to the P site. This is an iterative process that repeats itself until the ribosome at the termination codon.
Transcription And Translation: Ap® Biology Crash Course
Termination occurs when the A site of the ribosome reaches one of three codons (UAA, UAG or UGA).
In prokaryotes, these codons are recognized by differential transcription (abbreviated as RF). RF-1 is responsible for recognizing the termination codons UAA and UAG, while RF-2 – UGA and UAA. When these release factors bind to the complex, it causes the hydrolysis of the bond connecting the peptide to the tRNA in the P site and releases the nascent protein from the ribosome. Then the third release (RF-3-GTP) causes the release of RF-1 or RF-2 because GTP is hydrolyzed to GDP and a single phosphate residue. In contrast, eukaryote cells have only one transcription factor, eRF, which can recognize all three termination codons. The second factor is included – eRF-3, with the same function as RF-3 in prokaryotic cells. The process of protein synthesis in prokaryotes is summarized in the figure below. Some of the antibiotic inhibitors that may be involved in different protein synthesis steps are:
Related Topics Protein Synthesis Process Formation of Protein Targeting Polysomes Regulation of Translation Protein Synthesis Process Described with Pictures Protein biosynthesis begins with transcription and post-transcriptional modification in the nucleus. The mature mRNA is exported to the cytoplasm where it is transcribed. Polypeptide chains are folded and modified after translation.

Protein biosynthesis (or protein synthesis) is a basic biological process, occurring in the cell, balancing the loss of cellular proteins (through damage or export) through the production of new proteins. Proteins perform many important functions such as zymes, structural proteins or hormones. Protein synthesis is a similar process in both prokaryotes and eukaryotes but with distinct differences.
Synthesis Of Protein
Protein synthesis can be divided into two steps – translation and transcription. During transcription, the portion of DNA that encodes a protein, known as ge, is converted into a template molecule called messenger RNA (mRNA). This conversion is carried out by zymes, known as RNA polymerase, in the nucleus of the cell.
In eukaryotes, this mRNA is produced in an early form (pre-mRNA) that undergoes post-transcriptional modification to produce mature mRNA. Mature mRNA is exported from the cell nucleus through nuclear pores to the cytoplasm of the cell for translation. During translation, mRNA is read by ribosomes that use the nucleotide sequence of the mRNA to determine the sequence of amino acids. Ribosomes catalyze the formation of covalent peptide bonds between encoded amino acids to form polypeptide chains.
After translation, the polypeptide chain must fold to form a functional protein; for example, to function as a zyme the polypeptide chain must be properly folded to produce a functional active site. To form a functional three-dimensional (3D) structure, a polypeptide chain must form a set of smaller basic structures called secondary structures. The polypeptide chains in these secondary structures fold to produce the entire 3D hierarchical structure. Once the protein is properly folded, it can further evolve through various post-translational modifications. Post-translational modifications can change a protein’s ability to function, where it is located within the cell (eg cytoplasm or nucleus) and the protein’s ability to interact with other proteins.
Protein biosynthesis plays an important role in disease because changes and defects in this process, through DNA mutations or protein deficiency, are often the basis of disease. A DNA mutation changes the sequence of the next mRNA, which changes the amino acid sequence of the mRNA code. Mutations can make polypeptide chains shorter by activating stop sequences that cause early translation termination. Alternatively, a mutation in the mRNA sequence changes the amino acid specified at that position in the polypeptide chain. This amino acid change can affect the protein’s ability to function or fold properly.
Protein Synthesis (translation And Regulation Of Translation).
Misfolded proteins are often implicated in disease because improperly folded proteins have tdcy to stick together to form protein clumps. These clumps are linked to various diseases, often neurological, including Alzheimer’s disease and Parkinson’s disease.
Transcription occurs in the nucleus using DNA as a template to produce mRNA. In eukaryotes, this mRNA molecule is known as pre-mRNA because it undergoes post-transcriptional modification in the nucleus to produce a mature mRNA molecule. However, in prokaryotes post-transcriptional modification is not required so mature mRNA molecules are produced immediately upon transcription.
It shows the structure of the nucleotide with the 5 carbons labeled showing the 5′ nature of the phosphate group and the 3′ nature of the hydroxyl group needed to form a connective phosphodiester bond.

It shows the sequence of a DNA molecule with the coding strand 5′ to 3′ and the complementary template strand running 3′ to 5′
Translation: Making Protein Synthesis Possible
First, an enzyme called a helicase acts on the DNA molecule. DNA has an antiparallel structure, a double helix that forms two polynucleotide strands, linked together, with hydrogen bonds between the base pairs. The helicase disrupts the hydrogen bonds causing the region of the DNA – corresponding to the ge – to relax, separating the two DNA strands and exposing a set of bases. Although DNA is a double-stranded molecule, only one of the strands serves as a template for pre-mRNA synthesis – this strand is known as the template strand. The other DNA strand (which is complementary to the template strand) is known as the reverse strand.
Both DNA and RNA have intrinsic orientations, meaning that there are two distinct ds in the molecule. This directionality is due to the symmetry of the nucleotide subunits, with a phosphate group on one side of the ptosis sugar and a base on the other. The five carbons in the ptosis sugar are numbered from 1′ (which means prime) to 5′. Thus, the phosphodiester bond linking nucleotides is formed by linking the hydroxyl group on the 3′ carbon of one nucleotide to the phosphate group on the 5′ carbon of another nucleotide. Therefore, the DNA strand runs in the 5′ to 3′ direction and the complementary DNA strand runs in the opposite direction from 3′ to 5′.
RNA polymerase zyme binds to the exposed template strand and reads from the ge in
Where is the site of protein synthesis, cell free protein synthesis, cell free protein synthesis kit, protein synthesis in the cell, the site of protein synthesis in the cell is, the site of protein synthesis is the, protein synthesis in cell, site of protein synthesis, what is the site of protein synthesis in the cell, what is the site of protein synthesis, which is the site of protein synthesis, site of protein synthesis in the cell