Is Carbon Dioxide An Element Compound Or Mixture – The periodic table is made up of elements but these can combine with other elements to form compounds Sometimes they are found together but not bonded so we call them a mixture Let us now look at some examples of these meanings
Elements – An element is an atom or group of atoms that have the same number of protons Remember protons are the subatomic particles in the nucleus and any atom with the same number of protons is the same element. For example if I have a group of atoms that have all 6 protons, if I look at the periodic table I would say it would be the element carbon.
Is Carbon Dioxide An Element Compound Or Mixture

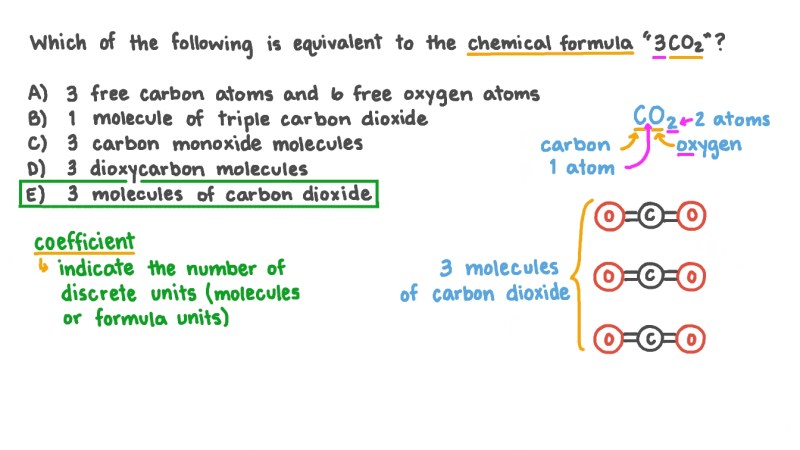
Compound – A compound is when two or more elements are bound together When we bond elements they exchange electrons and attract each other This means they are joined together to form a compound For example if we take carbon and react it with oxygen we get the compound carbon dioxide. This is when 2 oxygen atoms are bonded to a carbon atom
Examples Of Mixtures And Compounds
Mixture – A mixture is when we have elements or compounds in a container or space and they are not bound together. This means they do not bond and do not exchange or share electrons Instead, they do not react together but move around each other An example of this is the air in the classroom This air is a mixture of oxygen, carbon and nitrogen
Nathan holds a BSc in Biochemical Chemistry from the University of Warwick and a PGCE in Science from the University of Wolverhampton, UK. Nathan’s subjects range from general chemistry to organic chemistry Nathan also created a course on Breaking Atoms on the course page
René Descartes was a famous 11th century mathematician and philosopher who hypothesized the corpuscularism theory of atoms.
Semiconductors is a term used to describe a metal that is capable of conducting a current when an electrical force is applied due to the movement of electrons but the conductivity is not as high as that of metals due to fewer electrons to form a charge or low order structure.
Classify The Following Into Elements, Compounds And Mixtures. (a) Sodium (b) Soil (c) Sugar Solution (d) Silver (e) Calcium Carbonate (f) Tin (g) Silicon (h) Coal (i) Air (j) Soap (k) Methane (
An ionic compound is a bond that forms between a metal and a nonmetal to form a larger ionic lattice.
Nuclear fusion is a process that occurs in the Sun Under extreme heat and pressure, hydrogen atoms are forced together to form larger atoms of helium.
Heisenberg’s uncertainty principle is used to describe the relationship between the momentum and position of an electron. where the motion will be uncertain if the exact position of the electron is known

Werner Heisenberg was a German physicist who pioneered the field of quantum mechanics. He formulated the principle of uncertainty associated with the motion and position of an electron
What Is A Mixture In Chemistry? Definition And Examples
Lobes indicate the shape of the electron wave and the region of maximum probability of finding that electron as a particle.
The Pauli exclusion principle states that each electron can have only a unique set of 4 quantum numbers and that no two electrons can have the same quantum number.
Quantum number is a term used to describe electrons as a mathematical function to describe their motion and energy.
The term quantum mechanics refers to energy levels and the theoretical area of physics and chemistry where mathematics is used to explain the behavior of subatomic particles.
Elements, Compounds, And Mixtures Interactive Worksheet
Vibrational mode is a term used to describe stable motion in a molecule Generally these are vibrations, rotations and translations
Erwin Schrödinger was an Austrian physicist who used mathematical models to enhance the Bohr model of the electron and created an equation to predict the probability of finding an electron in a given state.
Corrosive metals, in Group 1 of the periodic table (formally known as Group IA), are so reactive that they are usually found in nature in combination with other elements. Alkali metals are shiny, soft, highly reactive metals at standard temperatures and pressures.

The alkaline earth metals are the second most reactive group in the periodic table They are found in Group 2 of the periodic table (formally known as Group IIA).
Carbon Dioxide Global Market Size, Share, Industry Report, Business Forecast 2032
The unknown elements (or transactinides) are the heaviest elements in the periodic table These are Meternium (Mt, atomic number 109), Darmstadium (Ds, atomic number 110), Roentanium (Rg, atomic number 111), Nihonium (Nh, atomic number 113), Muscovium (Mc, atomic number 115), Livermorium (Lv , atomic number 116) and tennessine (Ts, atomic number 117).
Transition metals are those found between transition metals (left) and metals (right). They include aluminum (Al), gallium (Ga), indium (In), thallium (Tl), tin (Sn), lead (Pb) and bismuth (B).
Organocene (Og) is a radioactive element with atomic number 118 in the periodic table, its form is not fully known due to the minuscule amount it produces. It is in group 18, with the symbol Og
Tenesine (Ts) is a radioactive element with atomic number 117 in the periodic table, its form is not fully known due to its production minus the amount. It is in Group 17 It has the symbol Ts
Pure Substances, Mixtures, Elements, And Compounds
Livermorium (Lv) is a radioactive element with atomic number 116, its form is not fully known due to its production minus its mass. It is in group 16, it has symbol Lv
Muscovium (Mac) is a radioactive metal with atomic number 115 115 in the periodic table, its form is not fully known due to its production minus the amount. It is in group 15, has the symbol MAC
Fluorovium (Fl) is a radioactive metal with atomic number 114, its form is completely unknown due to its origin minus the amount. It is in group 14, with the symbol Fl

Nihonium (Nh) is a radioactive metal with atomic number 112 in the periodic table, its form is not fully known due to the minuscule amount it originates from. It is in group 13, with the symbol Nh
Lesson Video: Compounds And Mixtures
Coppericium (Cr) is a radioactive metal with atomic number 112, its form is not fully known due to its production minus the amount. It is a transition metal in group 11 It has the symbol Rg
Roentgenium (Rg) is a radioactive metal with atomic number 111 in the periodic table, its form is not fully known due to its origin minus the amount. It is a transition metal in group 11 It has the symbol Rg
Darmstadium (Ds) is a radioactive metal with atomic number 110 in the periodic table, its form is not fully known due to its production minus the amount. It is a transition metal in group 10 It has the symbol Ds
Metaneurium (Mt) is a radioactive metal with atomic number 109 in the periodic table, its form is not fully known due to its abundance minus its abundance. It is a transition metal in group 9
Solved Classify Each Type Of Matter As An Element, A
Hasium (Hs) is a radioactive metal with atomic number 108 in the periodic table, its form is not fully known due to its production minus the amount. It is a transition metal in group
Borium (Bh) is a radioactive metal with atomic number 107 on the periodic table, its form is not fully known due to its abundance minus its abundance. It is a transition metal in group 7 It has Bh symbol
Seborgium (Sg) is a radioactive metal with atomic number 106 in the periodic table, its form is not fully known due to the minuscule amount it produces. It is a transition metal in group II It has the symbol Sg

Dubonium (Db) is a radioactive metal with atomic number 105 in the periodic table, its form is not fully known due to the small amount of minus produced. It is a transition metal in group 5 It has the symbol Db
Definition Of Compounds & Elements
Rutherfordium (RF) is a radioactive metal with atomic number 104, its form is not fully known due to its low abundance in the periodic table. It is a transition metal in group
Lawrencium (Lr) is a silvery-white radioactive metal with atomic number 103 in the periodic table. It is an actinoid metal with the symbol Lr
Nobelium (No) is a radioactive metal with atomic number 102 in the periodic table, its form is not fully known due to its production minus the amount. It is an actinoid metal with the number
Mendelevium (Md) is a radioactive metal with atomic number 101, its form is not fully known due to its origin minus its mass. It is an actinoid metal with the symbol Md
What’s The Difference Between Elements, Compounds And Mixtures?
Fermium (Fm) is a silver-white radioactive element
Is ink an element compound or mixture, is air an element compound or mixture, carbon dioxide mixture or compound, is gold an element compound or mixture, is iron an element compound or mixture, is water an element compound or mixture, is carbon dioxide an element or compound, carbon dioxide compound or element, is mercury an element compound or mixture, element compound or mixture, is lead an element compound or mixture, is glass an element compound or mixture