- How To Tell If A Reaction Is Endothermic Or Exothermic
- Does A Negative Deltah Correspond To An Endothermic Or Exothermic Process?
- Endothermic Or Exothermic Reaction?
- Can Someone Explain How Something Getting Cooler Is Endothermic?
- Compound Interest: Reversible Reactions, Equilibrium, And Le Chatelier’s Principle
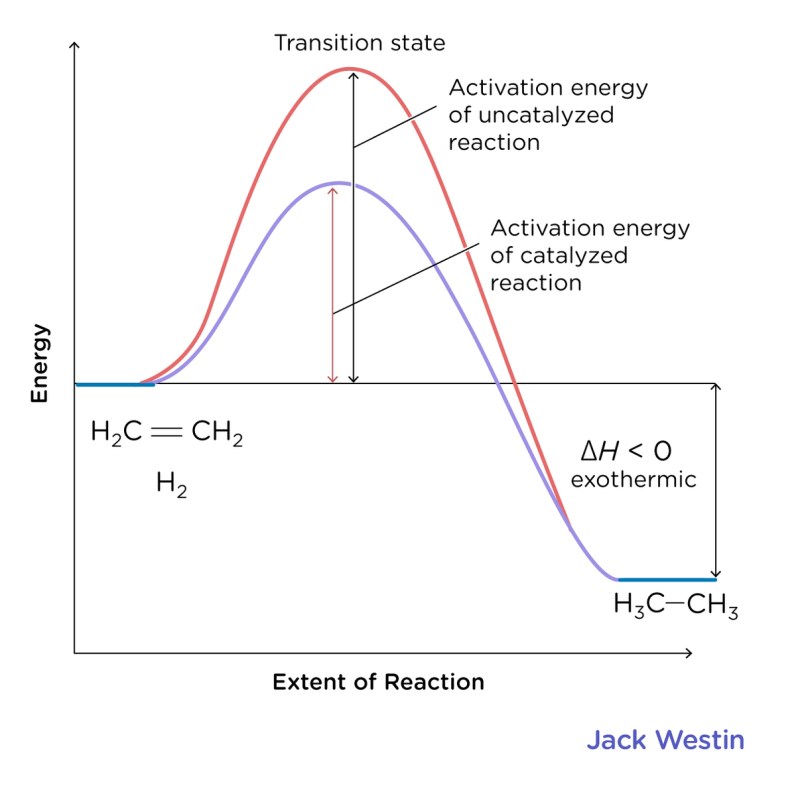
How To Tell If A Reaction Is Endothermic Or Exothermic – A high-energy diagram can be used to represent the energy changes that occur during a metabolic process. They compare the strength of the ingredients to the strength of the products. The energy difference between the two represents the amount of energy transferred to or absorbed from the environment. You should be able to draw and explain high energy models that represent endothermic and exothermic reactions.
In an endothermic reaction, heat energy is absorbed from the environment. Reactants start at a low energy level. As the energy of the heat increases, in response, the products are at a higher level of energy. This means that imitations will be less powerful than products. A typical energy level diagram for an endothermic reaction is shown below:
How To Tell If A Reaction Is Endothermic Or Exothermic

The energy difference between the reactants and products is the amount of heat energy absorbed from the environment. ▽H is represented in the diagram.
Answered: Stored Chemical Energy E) How Does The…
In an emergency, heat energy is released to the environment. Fertilizers start at a high energy level but as the thermal energy dissipates, the products are at lower energy levels. Impressions are more powerful than products.
The difference in energy between the medical device and the product is the amount of heat energy transferred to the environment. DH is represented on the diagram.
In a chemical reaction, the atoms in the reactants rearrange to form new substances. In order for this change to occur, the bonds holding the atoms together in the reactants must be broken. Once the atoms are separated, they can form new bonds to form products.
It must provide enough energy to break the bonds that hold the atoms together in the solvent. This energy is absorbed by the reactants in the environment. Therefore, bond breaking is an endothermic process. When new bonds are created to make products, energy is released into the environment. So, making a commitment is a creative process.
Rs 3.10 The Roles Of Exothermy And Endothermy In Roasting
The amount of force required to break the bonds can be compared to the amount of force exerted when the bonds are made, to determine whether they will form. synchronous or endothermic reactions. If the amount of energy required to break bonds is greater than the amount of energy released when making new bonds, the reaction will be endothermic.
If the amount of energy required to break the bonds is less than the amount of energy released when making new bonds, the reaction will be exothermic. and the term “Gibbs free energy” refers to biological processes. Links will take you to topics in general chemistry that you can read to refresh these ideas. This article is about making these principles easier to understand.
For starters, let’s answer this simple question: What will happen if we push the ball just to push it from the field to the mountain?

We know it will roll down the hill and there are many reasons that can explain why that happens, remember for the rest of your chemistry course it happens because the ball goes to a position low energy.
Does A Negative Deltah Correspond To An Endothermic Or Exothermic Process?
Any process that is considered a reaction or a simple physical process usually takes place at a low energy level. Falling anything, turning the sand watch, avalanche are a few examples of the process going into a low energy state.
If not disturbed, they tend to move in a direction that is characterized by low energy.
So, what is our problem here? Stress means that if we want the system to go to a higher energy level, then we need to provide the necessary energy to achieve that. Back to the ball, if we want to go up, we have to push it up or lift it up which means we get external force.
The meaning of all this is that the lower the energy means the higher the stability and vice versa – the higher the energy the more unstable the system.
Endothermic Or Exothermic Reaction?
Therefore, molecules or molecular compounds with lower energy are more stable and less active while those with higher energy are less stable and more reactive.
Molecules are not different from other systems because they have mechanical energy as a type of energy that arises from the electrical forces between nuclei and electrons. This energy changes during a chemical reaction and increases or decreases depending on the potential energy of the starting materials and products.
The energy change of a chemical reaction is shown using energy diagrams that relate to the reaction coordinate (time, rate front) and the strength of the starting materials and products.
These curves and data may not look as clear as the graphs with the ball because the terms enthalpy and free energy are often labeled instead of just stating the energy on the y axis. However, keep in mind that both are used to give the same information and that is the energy change in the process/reaction.
Can Someone Explain How Something Getting Cooler Is Endothermic?
For example, the reaction below is an example of a nucleophilic reaction between chloromethane and hydroxide ion:
What we see on the picture is that the energy of the starting material is higher than the product of the reaction. This means that during the reaction, the molecules lose some energy. And this energy is lost in a form of heat and heat is equal to enthalpy, or perhaps it is more correct to say that enthalpy is equal to heat when pressure remains constant. Now, since many reactions take place under the same pressure, the heat released or absorbed by a chemical is expressed by the enthalpy change (ΔHo). The Δ (delta) usually means the difference or change and the superscript ° indicates that the measurements are made under standard conditions.
One of the important things that must be noted before we move forward, just because the answer has a tendency to go to the lowest level of energy, does not mean that it will happen. It’s like the ball didn’t roll down the hill until the first kick was given. This is the activation energy as you may remember from general chemistry.
We will talk about free energy and Gibbs free energy explaining specific and non-specific processes in the following articles. However, in this post, let’s focus more on enthalpy to avoid unnecessary confusion.
I Need Help
When the enthalpy decreases, it is written with a negative sign and when it increases, the positive sign is given right in front of its quantity.
You may sometimes get confused with the negative and positive signs of the enthalpy change and wonder why exothermic is negative and endothermic is positive.
For this, remember that we are always looking from the point of view of the system and in chemistry, the system is the reaction (of molecules). So, if they lose heat then ΔH should be given with a negative sign.

If we look at the process from the point of view of the environment, then we can say that the environment has gained heat and therefore, the enthalpy change would have been positive. So, remember that we are always looking from the point of view of the system which means the molecules / the reaction type. If they lose/release heat, it’s an emergency (negative ΔHo
Endothermic Reaction At Room Temperature Enabled By Deep Ultraviolet Plasmons
If the body loses heat, there is an endothermic reaction, and the enthalpy change is shown in a positive sign.
Up to now, we have defined the enthalpy change in chemical reactions with negative or positive signs depending on the release of heat or absorption by the system. However, there is an interesting question that you may be thinking:
Why is there a change in enthalpy during chemical reactions? Now, to answer this question, we must go back a little in general chemistry and remember that atoms have a high potential energy when they are free and not bound to each other. On the other hand, when there is a chemical bond formed between them, they lose some energy, which means they go to a more stable state.
It can lose power in the form of heat. The same amount of energy will be required to break the bond and produce two atoms (homolytic cleavage).
Compound Interest: Reversible Reactions, Equilibrium, And Le Chatelier’s Principle
Therefore, this is the H-H bond strength, and the strength required to break it is called the dissociation strength. On the contrary, for the continuous process, when H2 is done, we are talking about the heat of formation and the number, these two things are different only in their signs.
Just like the H-H bond, the bonds between all elements are characterized by a specific bond strength (strong bond).
Now, back to the question of why there is an enthalpy change during a chemical reaction, we can look at it as the difference between the binding energies of the starting materials and the products. Remember, a chemical reaction is a rearrangement of atoms – some bonds are broken, and new bonds are formed. Each of these bonds requires energy to be broken individually

Exothermic and endothermic reaction, how to tell if a reaction is endothermic or exothermic, how to determine whether a reaction is exothermic or endothermic, how to tell if a rash is an allergic reaction, what is exothermic and endothermic reaction, is photosynthesis an exothermic or endothermic reaction, endothermic reaction vs exothermic reaction, define exothermic and endothermic reaction, endothermic and exothermic reaction examples, endothermic and exothermic reaction experiments, is the reaction exothermic or endothermic, endothermic and exothermic reaction worksheet